uitsluitend voor onderzoeksdoeleinden
I-BET151 (GSK1210151A) BET remmer
Cat.Nr.S2780
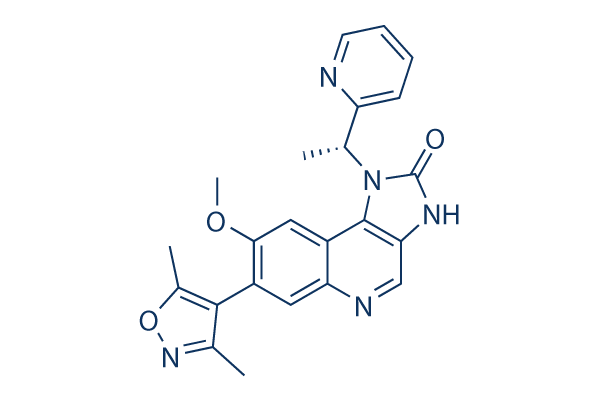
Chemische structuur
Moleculair gewicht: 415.44
Kwaliteitscontrole
| Gerelateerde doelwitten | HDAC JAK Histone Methyltransferase PKC PARP HIF PRMT EZH2 AMPK Histone Acetyltransferase |
|---|---|
| Overige BET Inhibitoren | Pelabresib (CPI-0610) ZEN-3694 (BET-IN-19) Molibresib (I-BET-762) Mivebresib (ABBV-075) INCB054329 Birabresib (OTX015) Apabetalone (RVX-208) AZD5153 6-hydroxy-2-naphthoic acid CPI-203 I-BRD9 |
Celkweek, behandeling & werkconcentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| MV4;11 | cytotoxicity assay | ~100 μM | DMSO | IC50=26 nM | 21964340 | |
| RS4;11 | cytotoxicity assay | ~100 μM | DMSO | IC50=192 nM | 21964340 | |
| MOLM13 | cytotoxicity assay | ~100 μM | DMSO | IC50=120 nM | 21964340 | |
| NOMO1 | cytotoxicity assay | ~100 μM | DMSO | IC50=15 nM | 21964340 | |
| HEL | cytotoxicity assay | ~100 μM | DMSO | IC50=1 μM | 21964340 | |
| K562 | cytotoxicity assay | ~100 μM | DMSO | IC50>100 μM | 21964340 | |
| MEG01 | cytotoxicity assay | ~100 μM | DMSO | IC50=25 μM | 21964340 | |
| HL60 | cytotoxicity assay | ~100 μM | DMSO | IC50=890 nM | 21964340 | |
| MV4;11 | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 21964340 | |
| MOLM13 | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 21964340 | |
| MV4;11 | Function assay | DMSO | decreases the recruitment of BRD3/4 and impaired recruitment of CDK9 and PAF1 to the transcriptional start site | 21964340 | ||
| PBMC | Function assay | DMSO | inhibits IL-6 with pIC50 of 6.7 | 22437115 | ||
| A2 | Function assay | ~10 μM | DMSO | reactivates latent HIV-1 | 23255218 | |
| A72 | Function assay | ~10 μM | DMSO | reactivates latent HIV-1 | 23255218 | |
| BC1 | Growth inhibitory assay | ~1 μM | DMSO | IC50=220 nM | 23792448 | |
| BC3 | Growth inhibitory assay | ~1 μM | DMSO | IC50=460 nM | 23792448 | |
| BCBL1 | Growth inhibitory assay | ~1 μM | DMSO | IC50=330 nM | 23792448 | |
| BJAB | Growth inhibitory assay | ~1 μM | DMSO | IC50=970 nM | 23792448 | |
| Namalwa | Growth inhibitory assay | ~1 μM | DMSO | IC50=970 nM | 23792448 | |
| Jurkat | Growth inhibitory assay | ~1 μM | DMSO | IC50=1220 nM | 23792448 | |
| MM1S | Growth inhibitory assay | ~1 μM | DMSO | IC50=760 nM | 23792448 | |
| U266 | Growth inhibitory assay | ~1 μM | DMSO | IC50=950 nM | 23792448 | |
| UM-PEL-1 | Growth inhibitory assay | ~1 μM | DMSO | IC50=210 nM | 23792448 | |
| UM-PEL-3 | Growth inhibitory assay | ~1 μM | DMSO | IC50=180 nM | 23792448 | |
| BC1 | Function assay | 500 nM | DMSO | induces cell-cycle arrest | 23792448 | |
| BC3 | Function assay | 500 nM | DMSO | induces cell-cycle arrest | 23792448 | |
| BC1 | Function assay | 800 nM | DMSO | reduces c-Myc protein levels | 23792448 | |
| BC3 | Function assay | 800 nM | DMSO | reduces c-Myc protein levels | 23792448 | |
| H929 | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| KMS12PE | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| KMS12BM | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| KMS18 | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| KMS11 | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| RPMI8226 | Function assay | ~1 μM | DMSO | induces cell cycle arrest | 24335499 | |
| H929 | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| KMS12PE | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| KMS12BM | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| KMS18 | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| KMS11 | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| RPMI8226 | Apoptosis assay | ~1 μM | DMSO | induces cell apoptosis | 24335499 | |
| U87MG | Function assay | ~10 μM | DMSO | reduces U87MG cellular ATP with IC50 of 1.05 μM | 24496381 | |
| A172 | Function assay | ~10 μM | DMSO | reduces cellular ATP with IC50 of 1.28 μM | 24496381 | |
| SW1783 | Function assay | ~10 μM | DMSO | reduces cellular ATP with IC50 of 2.68 μM | 24496381 | |
| U87MG | Function assay | ~10 μM | DMSO | increases proportion of cells in the G1/S transition | 24496381 | |
| RAW267.4 | Function assay | 1 μM | DMSO | reduces IL-6 production induced by LPS | 24859008 | |
| RAW267.4 | Function assay | 1 μM | DMSO | reduces the association between BRD4 and acetylated p65 | 24859008 | |
| Me007 | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| SK-Mel-28 | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| Mel-RMU | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| Mel-JD | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| Mel-RM | Growth inhibitory assay | ~100 μM | DMSO | inhibits the growth | 24906137 | |
| Me007 | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| SK-Mel-28 | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| Mel-RMU | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| Mel-JD | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| Mel-RM | Apoptosis assay | ~100 μM | DMSO | induces apoptosis | 24906137 | |
| Me007 | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| SK-Mel-28 | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| Mel-RMU | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| Mel-JD | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| Mel-RM | Function assay | 10 μM | DMSO | induces cell cycle arrest by upregulation of p21 | 24906137 | |
| Me007 | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| SK-Mel-28 | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| Mel-RMU | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| Mel-JD | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| Mel-RM | Function assay | 10 μM | DMSO | upregulates proapoptotic and cell cycle arrest genes | 24906137 | |
| HepG2 | Function assay | 18 hrs | Upregulation of ApoA1 expression in human HepG2 cells assessed as concentration required to increase 70% of luciferase activity after 18 hrs by luciferase reporter gene assay, EC170 = 0.09 μM. | 22386529 | ||
| Raji | Function assay | 4 hrs | Inhibition of BRD4 in human Raji cells assessed as reduction of MYC expression after 4 hrs, IC50 = 0.13 μM. | 24900758 | ||
| MV4-11 | Growth inhibition assay | 72 hrs | Growth inhibition of human MV4-11 cells after 72 hrs by SRB assay, IC50 = 0.119 μM. | 25559428 | ||
| MM1S | Growth inhibition assay | 72 hrs | Growth inhibition of human MM1S cells after 72 hrs by SRB assay, IC50 = 0.299 μM. | 25559428 | ||
| HT-29 | Growth inhibition assay | 72 hrs | Growth inhibition of human HT-29 cells after 72 hrs by SRB assay, IC50 = 0.945 μM. | 25559428 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD4 BD1 (44 to 168 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, IC50 = 0.0317 μM. | 26080064 | ||
| MV4-11 | Cytotoxicity assay | 4 days | Cytotoxicity against human MV4-11 cells harboring MLL1 fusion gene assessed as growth inhibition after 4 days by CellTiter-Glo luminescent assay, IC50 = 0.162 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD4 BD2 (333 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, IC50 = 0.226 μM. | 26080064 | ||
| MOLM13 | Cytotoxicity assay | 4 days | Cytotoxicity against human MOLM13 cells harboring MLL1 fusion gene assessed as growth inhibition after 4 days by CellTiter-Glo luminescent assay, IC50 = 0.228 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE3 cells after 30 mins by Flourescence polarization assay, IC50 = 0.0317 μM. | 28463487 | ||
| MV4-11 | Growth inhibition assay | 4 days | Growth inhibition of human MV4-11 cells after 4 days by WST-8 assay, IC50 = 0.162 μM. | 28463487 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 DE3 cells after 30 mins by Flourescence polarization assay, IC50 = 0.226 μM. | 28463487 | ||
| MOLM13 | Growth inhibition assay | 4 days | Growth inhibition of human MOLM13 cells after 4 days by WST-8 assay, IC50 = 0.228 μM. | 28463487 | ||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD3 BD1 (24 to 144 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0298 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD3 BD2 (306 to 417 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0405 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD4 BD1 (44 to 168 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0528 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD2 BD1 (72 to 205 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0548 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD2 BD2 (349 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.0703 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated BRD4 BD2 (333 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 0.215 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | Binding affinity to biotinylated CREBBP (1043 to 1159 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells by bio-layer interferometry method, Kd = 3.084 μM. | 26080064 | |||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD3 BD1 (24 to 144 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.0072 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD4 BD1 (44 to 168 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.009 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD2 BD1 (72 to 205 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.009 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD3 BD2 (306 to 417 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.0223 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD2 BD2 (349 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.0496 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Displacement of FAM-labeled ZBA248 from BRD4 BD2 (333 to 460 amino acid residues) (unknown origin) expressed in Rosetta2 DE3 cells after 30 mins by fluorescence polarization assay, Ki = 0.0748 μM. | 26080064 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE3 cells after 30 mins by Flourescence polarization assay, Ki = 0.009 μM. | 28463487 | ||
| Rosetta2 DE3 | Function assay | 30 mins | Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 DE3 cells after 30 mins by Flourescence polarization assay, Ki = 0.0748 μM. | 28463487 | ||
| THP1 | Antiinflammatory assay | Antiinflammatory activity in human THP1 cells | 22386529 | |||
| HT-29 | Function assay | 0.3125 uM to 5 uM | 24 hrs | Inhibition of BRD4 in human HT-29 cells assessed as reduction in c-Myc protein expression at 0.3125 uM to 5 uM uM after 24 hrs by Western blotting method | 25559428 | |
| MV4-11 | Function assay | Inhibition of BRD4 in human MV4-11 cells assessed as downregulation of BCL2 RNA expression by RNA-seq analysis | 29259751 | |||
| MV4-11 | Function assay | Inhibition of BRD4 in human MV4-11 cells assessed as downregulation of cMYC RNA expression by RNA-seq analysis | 29259751 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| fibroblast cells | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for control Hh wild type fibroblast cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| Klik om meer experimentele gegevens over de cellijn te bekijken | ||||||
Chemische informatie, Opslag en Stabiliteit
| Moleculair gewicht | 415.44 | Formule | C23H21N5O3 |
Opslag (Vanaf de ontvangstdatum) | |
|---|---|---|---|---|---|
| CAS-nr. | 1300031-49-5 | SDF downloaden | Opslag van stamoplossingen |
|
|
| Synoniemen | N/A | Smiles | CC1=C(C(=NO1)C)C2=C(C=C3C(=C2)N=CC4=C3N(C(=O)N4)C(C)C5=CC=CC=N5)OC | ||
Oplosbaarheid
|
In vitro |
DMSO
: 83 mg/mL
(199.78 mM)
Ethanol : 83 mg/mL Water : Insoluble |
Molariteitscalculator
|
In vivo |
|||||
In vivo Formuleringscalculator (Heldere oplossing)
Stap 1: Voer de onderstaande informatie in (Aanbevolen: Een extra dier voor het geval van verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in het gedeelte Oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-mastervloeistof: mg geneesmiddel vooraf opgelost in μL DMSO ( Concentratie mastervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de partij geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toeμL PEG300, mengen en helder maken, voeg vervolgens toeμL Tween 80, mengen en helder maken, voeg vervolgens toe μL ddH2O, mengen en helder maken.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toe μL Maïsolie, mengen en helder maken.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysische methoden zoals vortexen, echografie of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Kenmerken |
Optimized to retain excellent BET target potency and selectivity while enhancing the in vivo pharmacokinetics and terminal half-life to enable prolonged in vivo studies.
|
|---|---|
| Targets/IC50/Ki |
BRD3
(Cell-free assay) 0.25 μM
BRD2
(Cell-free assay) 0.5 μM
BRD4
(Cell-free assay) 0.79 μM
|
| In vitro |
I-BET151 (GSK1210151A) vertoont een krachtige selectiviteit over een uitgebreid scala aan diverse eiwittypes, zoals COX-2, P450, Aurora B, GSK3β, PI3K-γ, GPCR, ionkanalen en transporters. Vergelijkbaar met I-BET762 (GSK525762A), vertoont het een krachtige bindingsaffiniteit voor BRD2, BRD3 en BRD4 met KD van 0,02-0,1 μM, en remt het significant de door lipopolysaccharide gestimuleerde IL-6-cytokineproductie in menselijke perifere bloedmononucleaire cellen (PBMC) en volbloed (WB) evenals ratten-WB met IC50 van respectievelijk 0,16 μM, 1,26 μM en 1,26 μM. Deze verbinding (0,5 of 5 μM) remt de binding van BETs (BRD2, BRD3, BRD4 en BRD9), maar niet de binding van 23 andere bromodomeine-eiwitten in HL60 nucleair extract aan geacetyleerde histonpeptiden. Het heeft een krachtige werkzaamheid tegen cellijnen die verschillende MLL-fusies bevatten, zoals MV4;11, RS4;11, MOLM13 en NOMO1-cellen met IC50 van 15-192 nM. Consistent daarmee elimineert het volledig het kolonie-vormende potentieel van MLL-fusie-gestuurde leukemieën (MOLM13), maar niet leukemieën gestuurd door tyrosinekinase-activering (K562). I-BET151 vertoont ook een krachtige werkzaamheid in zowel vloeibare kweek- als clonogene assays met primaire muizenvoorlopercellen getransformeerd met MLL-ENL of MLL-AF9. Behandeling met deze verbinding induceert significant apoptose en prominente G0/G1-arrestatie in MLL-fusie-cellijnen aangedreven door verschillende MLL-fusies (MOLM13 en MV4;11 die respectievelijk MLL-AF9 en MLL-AF4 bevatten), maar niet de K562-cellen, waarschijnlijk vanwege de remming van de transcriptie van BCL2, C-MYC en CDK6 door het blokkeren van de rekrutering van BRD3/4, PAFc en SEC-componenten naar de transcriptionele startplaats (TSS).
|
| Kinase Assay |
Fluorescentie-anisotropie (FP) ligandverplaatsingsassay
|
|
Alle componenten worden opgelost in buffer van samenstelling 50 mM HEPES pH 7.4, 150 mM NaCl en 0.5 mM CHAPS met eindconcentraties van BRD 2/3/4 75 nM, fluorescerend ligand 5 nM. 10 μL van dit reactiemengsel wordt met een micro multidrop toegevoegd aan putjes die 100 nL van verschillende concentraties I-BET151 (GSK1210151A) of DMSO-vehiculum (1% finaal) bevatten in een Greiner 384-wells zwarte microtiterplaat met laag volume en geëquilibreerd in het donker gedurende 60 minuten bij kamertemperatuur. Fluorescentie-anisotropie wordt afgelezen in Envision (lex = 485 nm, lEM = 530 nm; Dichroïsch = 505 nM).
|
|
| In vivo |
I-BET151 (GSK1210151A), toegediend met 30 mg/kg/dag, remt significant de tumorgroei van muizen MLL-AF9 en menselijke MLL-AF4 leukemie bij muizen, en biedt een aanzienlijk overlevingsvoordeel.
|
Referenties |
Toepassingen
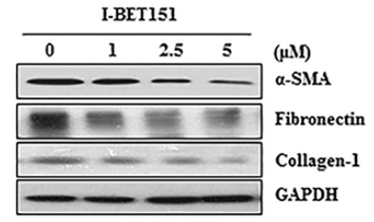
| Methoden | Biomarkers | Afbeeldingen | PMID |
|---|---|---|---|
| Western blot | α-SMA / Fibronectin / Collagen-1 FoxM1 / AURKB / Survivin / cyclin B / PLK1 HP1α / HP1β / HP1γ |
|
27732564 |
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, kunt u een bericht achterlaten.
