uitsluitend voor onderzoeksdoeleinden
Cilengitide trifluoroacetate Integrin remmer
Cat.Nr.S7077
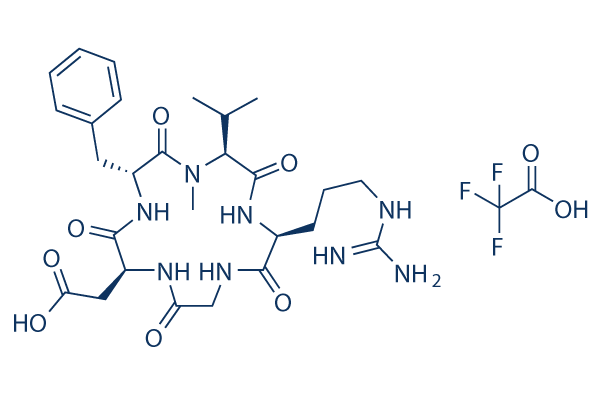
Chemische structuur
Moleculair gewicht: 702.68
Kwaliteitscontrole
| Gerelateerde doelwitten | Akt Wnt/beta-catenin PKC HSP ROCK Microtubule Associated Bcr-Abl Actin FAK Kinesin |
|---|---|
| Overige Integrin Inhibitoren | SB273005 Cilengitide (EMD 121974) RGD peptide (Arg-Gly-Asp) ATN-161 Cyclo(-RGDfK) TFA Cyclo(RGDyK) TFA Leukadherin-1 A-205804 Pyrintegrin RGD peptide (GRGDNP) |
Celkweek, behandeling & werkconcentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| G28 | Function Assay | 50 μg/ml | 30/60/120 min | inhibits phosphorylation of FAK, Src and Akt | 19114005 | |
| HMEC-1 | Function Assay | 20/40/60 μg/ml | inhibits FAK and Src | 19114005 | ||
| G44 | Apoptosis Assay | 1/5/50 μg/ml | 24 h | induces apoptosis | 19114005 | |
| G28 | Apoptosis Assay | 1/5/50 μg/ml | 24 h | induces apoptosis | 19114005 | |
| G44 | Proliferation Assay | 1/5/50 μg/ml | 24/48/72 h | inhibits proliferation in a dose dependent manner | 19114005 | |
| G28 | Proliferation Assay | 1/5/50 μg/ml | 24/48/72 h | inhibits proliferation in a dose dependent manner | 19114005 | |
| HMEC-1 | Apoptosis Assay | 1/5/50 μg/ml | 24 h | induces apoptosis | 19114005 | |
| HMEC-1 | Proliferation Assay | 1/5/50 μg/ml | 24/48/72 h | inhibits proliferation in a dose dependent manner | 19114005 | |
| HMEC-1 | Function Assay | 1/5/50 μg/ml | 24 h | induces a dose dependent detachment | 19114005 | |
| LNT-229 | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| T98G | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| LN-18 | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| LN-308 | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| U87MG | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| U87 | Function Assay | 0-25 μg/mL | 12 h | induces autophagy dose dependently | 21788343 | |
| U251 | Function Assay | 0-25 μg/mL | 12 h | induces autophagy dose dependently | 21788343 | |
| U87 | Apoptosis Assay | 25 μg/mL | 24/48 h | induces apoptosis at 48 h significantly | 21788343 | |
| U251 | Apoptosis Assay | 25 μg/mL | 24/48 h | induces apoptosis at 48 h significantly | 21788343 | |
| U87 | Growth Inhibition Assay | 0-25 μg/mL | 0-48 h | inhibits cell growth in dose and time dependent manner | 21788343 | |
| U251 | Growth Inhibition Assay | 0-25 μg/mL | 0-48 h | inhibits cell growth in dose and time dependent manner | 21788343 | |
| U251MG | Apoptosis Assay | 1 µM | 48 h | induces apoptosis | 23354807 | |
| U87MG | Apoptosis Assay | 1 µM | 48 h | induces apoptosis | 23354807 | |
| U251MG | Growth Inhibition Assay | 0-25 μM | 24/48 h | inhibits cell growth in dose and time dependent manner | 23354807 | |
| U87MG | Growth Inhibition Assay | 0-25 μM | 24/48 h | inhibits cell growth in dose and time dependent manner | 23354807 | |
| MCF-7 | Apoptosis Assay | 0-20 μM | 48 h | induces apoptosis | 24153102 | |
| T-47D | Apoptosis Assay | 0-20 μM | 48 h | induces apoptosis | 24153102 | |
| MCF-7 | Growth Inhibition Assay | 0-20 μM | 96 h | inhibits cell growth in a dose dependent manner | 24153102 | |
| T-47D | Growth Inhibition Assay | 0-20 μM | 96 h | inhibits cell growth in a dose dependent manner | 24153102 | |
| FaDu | Apoptosis Assay | 25 µM | 48 h | induces apoptosis | 24557056 | |
| CAL27 | Apoptosis Assay | 25 µM | 48 h | induces apoptosis | 24557056 | |
| SCC25 | Apoptosis Assay | 25 µM | 48 h | induces apoptosis | 24557056 | |
| FaDu | Growth Inhibition Assay | 6.25–200 µM | 72 h | results moderate, dose-dependent growth inhibition | 24557056 | |
| CAL27 | Growth Inhibition Assay | 6.25–200 µM | 72 h | results moderate, dose-dependent growth inhibition | 24557056 | |
| SCC25 | Growth Inhibition Assay | 6.25–200 µM | 72 h | results moderate, dose-dependent growth inhibition | 24557056 | |
| H28 | Cell Viability Assay | 1 nM-200 μM | 72 h | decreases cell viability in a dose dependent manner | 24595274 | |
| MM05 | Cell Viability Assay | 1 nM-200 μM | 72 h | decreases cell viability in a dose dependent manner | 24595274 | |
| MSTO-211H | Cell Viability Assay | 1 nM-200 μM | 72 h | decreases cell viability in a dose dependent manner | 24595274 | |
| REN | Cell Viability Assay | 1 nM-200 μM | 72 h | decreases cell viability in a dose dependent manner | 24595274 | |
| LN-308 | Function Assay | 10 μm | 24 h | DMSO | reduces AhR protein levels and DRE reporter activity | 26500056 |
| HaCaT | Function Assay | 10 μm | 48 h | DMSO | reduces TGF-β2 mRNA expression | 26500056 |
| S-24 | Function Assay | 1/10 μm | 24 h | DMSO | reduces DRE reporter activity | 26500056 |
| ZH-161 | Function Assay | 1/10 μm | 24 h | DMSO | reduces DRE reporter activity | 26500056 |
| LN-308 | Function Assay | 1/10/100 μm | 24 h | DMSO | reduces DRE reporter activity in a concentration-dependent manner | 26500056 |
| M21 | Function assay | 1 hr | Binding affinity to integrin alphav/beta3 heterodimer in human M21 cells assessed as inhibition of integrin-mediated human M21 cell adhesion to vitronectin after 1 hr in presence of MnCl2, IC50 = 0.0004 μM. | 26753814 | ||
| HEK293T | Function assay | 2 hrs | Binding affinity to soluble truncated human recombinant Fc-tagged alphaVbeta3 and integrins were expressed in HEK293T cells after 2 hrs by competition ELISA-like assay, IC50 = 0.00051 μM. | 24095096 | ||
| HT-29 | Function assay | 2 hrs | Antagonist activity at integrin alphaVbeta5 (unknown origin) expressed in HT-29 cells assessed as reduction in cell adhesion to vitronectin after 2 hrs by MTT assay, IC50 = 0.12 μM. | 28351594 | ||
| HEK293 | Function assay | 2 hrs | Antagonist activity at integrin alphaVbeta3 (unknown origin) expressed in HEK293 cells assessed as reduction in cell adhesion to fibrinogen after 2 hrs by MTT assay, IC50 = 0.22 μM. | 28351594 | ||
| SKOV3 | Function assay | 2 hrs | Antagonist activity at integrin alphaVbeta3alphaVbeta5 (unknown origin) expressed in SKOV3 cells assessed as reduction in cell adhesion to fibrinogen after 2 hrs by MTT assay, IC50 = 0.37 μM. | 28351594 | ||
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in p53 accumulation at 100 nM after 24 hrs by Western blot method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in p53 accumulation at 100 nM after 24 hrs in presence of MDM2 inhibitor nutlin-3 by Western blot method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in p53 accumulation at 100 nM after 24 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by Western blot method | 29775303 | |
| U87MG | Function assay | 100 nM | 8 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in MDM2 mRNA expression at 100 nM after 8 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in PUMA mRNA expression at 100 nM after 24 hrs by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in PUMA mRNA expression at 100 nM after 24 hrs in presence of MDM2 inhibitor nutlin-3 by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in PUMA mRNA expression at 100 nM after 24 hrs in presence of MDM4 inhibitor SJ-1722550 by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in PUMA mRNA expression at 100 nM after 24 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in p21 mRNA expression at 100 nM after 24 hrs by RT-PCR method | 29775303 | |
| U87MG | Antiproliferative assay | 100 nM | 72 hrs | Antiproliferative activity against human U87MG cells at 100 nM after 72 hrs in presence of MDM2 inhibitor nutlin-3 by MTS assay | 29775303 | |
| U87MG | Antiproliferative assay | 100 nM | 72 hrs | Antiproliferative activity against human U87MG cells at 100 nM after 72 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by MTS assay | 29775303 | |
| U87MG | Cell cycle assay | 100 nM | 24 hrs | Cell cycle arrest in human U87MG cells assessed as accumulation at G0/G1 phase at 100 nM after 24 hrs | 29775303 | |
| U87MG | Anti-invasive assay | 10 uM | 24 hrs | Anti-invasive activity in human U87MG cells at 10 uM after 24 hrs by transwell assay | 29775303 | |
| U87MG | Anti-invasive assay | 10 uM | 24 hrs | Anti-invasive activity in human U87MG cells at 10 uM after 24 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by transwell assay | 29775303 | |
| Klik om meer experimentele gegevens over de cellijn te bekijken | ||||||
Chemische informatie, Opslag en Stabiliteit
| Moleculair gewicht | 702.68 | Formule | C29H41F3N8O9 |
Opslag (Vanaf de ontvangstdatum) | |
|---|---|---|---|---|---|
| CAS-nr. | 199807-35-7 | SDF downloaden | Opslag van stamoplossingen |
|
|
| Synoniemen | EMD 121974, NSC 707544 | Smiles | CC(C)C1C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC(C(=O)N1C)CC2=CC=CC=C2)CC(=O)O)CCCN=C(N)N.C(=O)(C(F)(F)F)O | ||
Oplosbaarheid
|
In vitro |
DMSO
: 100 mg/mL
(142.31 mM)
Water : 6.25 mg/mL Ethanol : Insoluble |
Molariteitscalculator
|
In vivo |
|||||
In vivo Formuleringscalculator (Heldere oplossing)
Stap 1: Voer de onderstaande informatie in (Aanbevolen: Een extra dier voor het geval van verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in het gedeelte Oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-mastervloeistof: mg geneesmiddel vooraf opgelost in μL DMSO ( Concentratie mastervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de partij geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toeμL PEG300, mengen en helder maken, voeg vervolgens toeμL Tween 80, mengen en helder maken, voeg vervolgens toe μL ddH2O, mengen en helder maken.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toe μL Maïsolie, mengen en helder maken.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysische methoden zoals vortexen, echografie of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Targets/IC50/Ki |
αvβ3 receptor
(Cell-free assay) 4.1 nM
αvβ5 receptor
(Cell-free assay) 79 nM
|
|---|---|
| In vitro |
Cilengitide is een gecycliseerd pentapeptide-peptidomimeticum dat is ontworpen om te concurreren met de arginine-glycine-asparaginezuur (RGD) peptidesequentie die de integrin-ligandbinding reguleert. Cilengitide blokkeert selectief en potent de ligatie van de αvβ3- en αvβ5-integrinen aan provisionele matrixeiwitten zoals vitronectine, fibronectine, fibrinogeen, von Willebrand-factor, osteopontine en andere.
Cilegitide remt angiogenese in vitro. 10 μM Cilengitide remt de aanhechting van BAE-, BME- en HUVE-cellen op vitronectine en fibronectine volledig. Cilengitide remt de in vitro angiogenese van BAE-cellen op driedimensionale collageen- en fibrinegels die zijn voorbehandeld met FGF-2 (of VEGF-A) met IC50 van respectievelijk 15 μM en 8 μM, 4 μM en 3 μM.
Cilengitide blokkeert proliferatie en induceert apoptose van endotheelcellen, evenals differentiatie van menselijke endotheel precursorcellen (EPC's). 50 μg/mL Cilengitide remt de proliferatie van de menselijke microvasculaire endotheelcellijn HMEC-1 volledig en leidt tot apoptose in ~30% van de cellen. 1,0 μM Cilengitide gedurende 9 dagen remt de proliferatie van EPC's met bijna 40%. 1 μM Cilengitide remt de differentiatie van EPC's met meer dan 80% na 14 dagen.
Cilengitide remt adhesie en induceert apoptose van tumorcellen. 25 μg/mL Cilengitide veroorzaakt loslating van DAOY-cellen (medulloblastoom) en U87MG-cellen (glioblastoom) van vitronectine en tenascine met meer dan 60%. 25 μg/mL Cilengitide induceert een apoptosepercentage van bijna 50% van deze cellen.
|
| Kinase Assay |
Integrin-binding competitie assay
|
|
Recombinante oplosbare integrinen worden geïmmobiliseerd, en peptiden, die serieel verdund zijn in Tris-gebufferde zoutoplossing (TBS++) (0,1% (w/v) BSA, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2 10 μM MnCl2, 20 mM Tris-HCl; pH 7,4), worden parallel toegevoegd met gebiotinyleerd vitronectine (tot 1μg/mL). Na 3 uur incubatie bij 37℃ en wassen met Tris-gebufferde zoutoplossing, wordt gebonden ligand gedetecteerd door incubatie met een anti-biotine alkalische fosfatase-geconjugeerd antilichaam (BioRad) gevolgd door ontwikkeling met p-nitrofenylfosfatase substraat. De reactie wordt gestopt door toevoeging van NaOH en de kleurintensiteit wordt afgelezen bij 405 nm.
|
|
| In vivo |
Cilengitide is actief tegen tumorgroei en angiogenese als enkelvoudig middel. 100 μg Cilengitide induceert een significante afname van het aantal CD31+ vaten dat in tumoren wordt gezien (2/gezichtsveld met hoge vergroting) in vergelijking met controletumoren (56/gezichtsveld met hoge vergroting). 100 μg Cilengitide verhoogt cellulaire apoptose in de hersentumoren van dieren (2,2% apoptotische cellen/gezichtsveld met hoge vergroting) in vergelijking met die welke het inactieve peptide kregen (1,7% cellen/gezichtsveld met hoge vergroting). Cilengitide-behandeling resulteert in een verlengde overleving van muizen met melanoomxenografts M21 in vergelijking met de controlegroep (36,5 vs 17,3 dagen).
Cilengitide kan het therapeutische voordeel van cytotoxische middelen, waaronder chemotherapie en radiotherapie, in tumormodellen vergroten. Cilengitide (250 mg/dosis) alleen verandert de tumorgroei van borstkankerxenografts niet in vergelijking met onbehandelde muizen, maar gecombineerde modaliteit RIT (CMRIT) met behulp van RIT en zes doses Cilengitide (250 mg/dosis) verhoogt de werkzaamheid van de behandeling, waarbij het genezingspercentage voor muizen die alleen RIT krijgen, toeneemt van 15% naar 53%. CMRIT verhoogt significant de apoptose van tumor- en endotheelcellen na 5 dagen, en vermindert tumorproliferatie.
|
Referenties |
|
Toepassingen
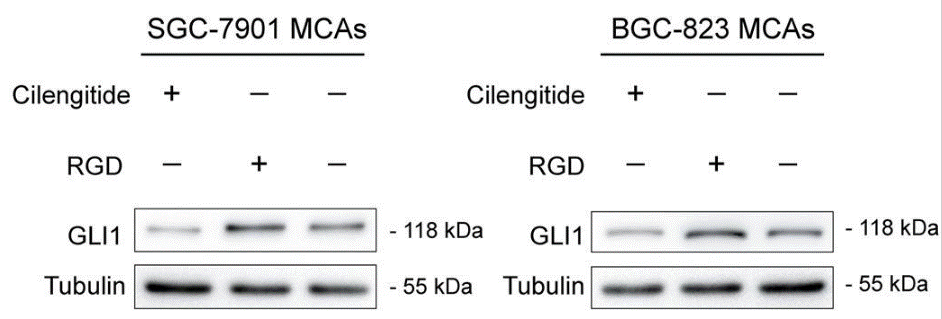
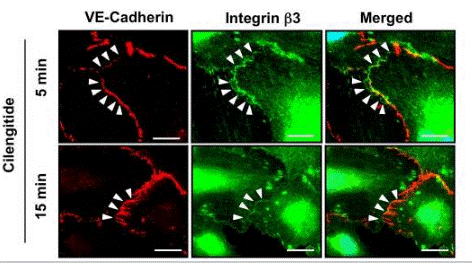
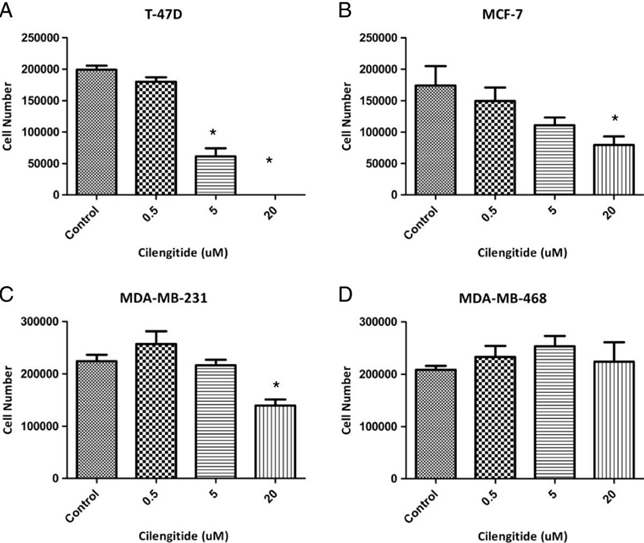
| Methoden | Biomarkers | Afbeeldingen | PMID |
|---|---|---|---|
| Western blot | GLI1 pFAK / p-AKT |
|
31366904 |
| Immunofluorescence | VE-cadherin / β3 integrin |
|
19212436 |
| Growth inhibition assay | Cell number |
|
24153102 |
Informatie klinische proef
(gegevens van https://clinicaltrials.gov, bijgewerkt op 2024-05-22)
| NCT-nummer | Rekrutering | Aandoeningen | Sponsor/Medewerkers | Startdatum | Fasen |
|---|---|---|---|---|---|
| NCT01517776 | Terminated | Gliomas |
Martin-Luther-Universität Halle-Wittenberg|Merck KGaA Darmstadt Germany |
January 2012 | Phase 2 |
| NCT01118676 | Completed | Locally Advanced Non Small Cell Lung Cancer (NSCLC) |
Institut Claudius Regaud|Merck KGaA Darmstadt Germany |
March 2010 | Phase 1 |
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, kunt u een bericht achterlaten.
Veelgestelde vragen
Vraag 1:
The recommended vehicle for it is 30% propylene glycol, 5% Tween 80, 65% D5W at 30mg/ml. Can you let me know if this is a suspension or clear solution?
Antwoord:
S7077 can be dissolved in 30% propylene glycol/5% Tween 80/65% D5W at 10 mg/ml as a clear solution.
Vraag 2:
Is it a TFA salt?
Antwoord:
S7077 is actually a TFA salt, and the ratio between it and TFA is 1:1.
