uitsluitend voor onderzoeksdoeleinden
Aspirin COX remmer
Cat.Nr.S3017
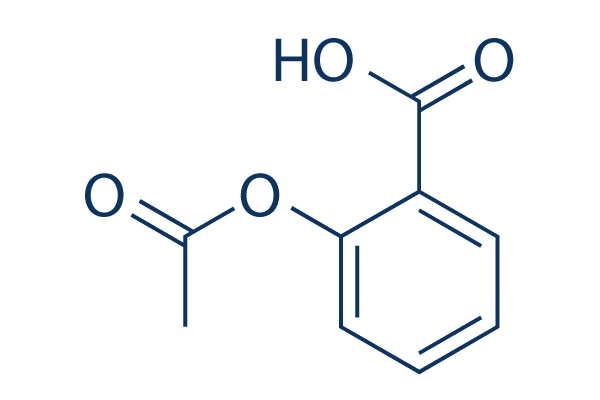
Chemische structuur
Moleculair gewicht: 180.16
Kwaliteitscontrole
| Gerelateerde doelwitten | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Overige COX Inhibitoren | NS-398 (NS398) Paeoniflorin (NSC 178886) Lumiracoxib Xanthohumol Bufexamac Nimesulide Ginsenoside Rd Pranoprofen Phenacetin Tolfenamic Acid |
Celkweek, behandeling & werkconcentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| AGS | Function assay | 15 to 60 umol/L after | 12 hrs | Inhibition of Escherichia coli-stimulated IL-8 production in human AGS cells at 15 to 60 umol/L after 12 hrs by ELISA | 20153183 | |
| microglia cells | Antineuroinflammatory assay | 15 mins | Antineuroinflammatory activity in LPS-stimulated rat microglia cells assessed as inhibition of PMA-stimulated TXB2 release preincubated for 15 mins measured 70 mins after PMA challenge, IC50=3.12μM | 22153874 | ||
| HCT116 | Function assay | 1 mM | 6 hrs | Inhibition of TNF-alpha-induced NF-kappaB activation in human HCT116 cells at 1 mM after 6 hrs by luciferase reporter gene assay | 22154834 | |
| SKBR3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human SKBR3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| PANC1 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PANC1 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| PC3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PC3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| MDA-MB-231 | Function assay | 100 uM | 30 mins | Irreversible inhibition of COX-1 in human MDA-MB-231 cells assessed as inhibition of arachidonic acid-induced PGE2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | 23651359 | |
| THP1 | Function assay | 100 uM | 30 mins | Irreversible inhibition of COX-1 in human THP1 cells assessed as inhibition of arachidonic acid-induced TXB2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | 23651359 | |
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as reduction in cell viability after 48 hrs MTT assay | 26750401 | ||
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-6 in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of TNF-alpha in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by E | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring reduction in MDA levels at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in SOD activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in catalase activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of TNF-alpha in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-1beta in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as reduction in MDA levels at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in SOD activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by spec | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-6 in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-1beta in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by EL | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced cytotoxicity in rat H9c2 cells assessed as increase in cell viability at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by MTT assay | 27575471 | |
| RAW264.7 | Antiinflammatory assay | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide production by measuring nitrite accumulation by Griess method, IC50=43.2μM | 28561586 | |||
| stem cells | Function assay | 100 uM | 5 days | Induction of adipogenesis in human bone marrow-derived mesenchymal stem cells assessed as increase in adiponectin production at 100 uM measured on day 5 in presence of IDX by ELISA | 29398443 | |
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| peritoneal cells | Function assay | 5 hrs | Inhibition of LPS-induced PGE2 production in C57BL6 mouse peritoneal cells measured at 5 hrs time interval by ELISA, IC50=4.08μM | 30006172 | ||
| HCT8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT8 cells after 72 hrs in presence of cinnamaldehyde by MTT assay, IC50=15.6μM | 30037494 | ||
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at G0/G1 phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at S phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as reduction in accumulation at G2/M phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| HaCaT | Antipyretic assay | 100 uM | 2 hrs | Antipyretic activity in human HaCaT cells assessed as inhibition of TNFalpha-induced PGE2 production at 100 uM pre-incubated for 2 hrs before TNFalpha stimulation for 24 hrs by ELISA | 31393125 | |
| OVCAR5 | Function assay | 300 uM to 1 mM | 72 hrs | Inhibition of NAPRT in human OVCAR5 cells assessed as potentiation of NAMPT inhibitor FK866-induced cytotoxicity by measuring reduction in cell viability at 300 uM to 1 mM incubated for 72 hrs by SRB assay | ChEMBL | |
| CCRF-CEM | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human CCRF-CEM cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| Jurkat | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Jurkat cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further i | ChEMBL | |
| ML2 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human ML2 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further incu | ChEMBL | |
| NOMO | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NOMO cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| NB4 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NB4 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further incu | ChEMBL | |
| NAMALWA | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NAMALWA cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| Daudi | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Daudi cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further in | ChEMBL | |
| Raji | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Raji cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| ARH77 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human ARH77 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further in | ChEMBL | |
| RPMI8226 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human RPMI8226 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| U266 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human U266 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| NAMALWA | Function assay | 35 mg/kg | 45 days | Potentiation of NAMPT inhibitor 10 mg/kg, ip bid FK866-induced antitumor activity in human NAMALWA cells xenografted in SCID mouse assessed as increase in mouse survival at 35 mg/kg, ip bid up to 45 days | ChEMBL | |
| Klik om meer experimentele gegevens over de cellijn te bekijken | ||||||
Chemische informatie, Opslag en Stabiliteit
| Moleculair gewicht | 180.16 | Formule | C9H8O4 |
Opslag (Vanaf de ontvangstdatum) | |
|---|---|---|---|---|---|
| CAS-nr. | 50-78-2 | SDF downloaden | Opslag van stamoplossingen |
|
|
| Synoniemen | NSC 27223, Acetylsalicylic acid, ASA | Smiles | CC(=O)OC1=CC=CC=C1C(=O)O | ||
Oplosbaarheid
|
In vitro |
DMSO
: 36 mg/mL
(199.82 mM)
Ethanol : 36 mg/mL Water : Insoluble |
Molariteitscalculator
|
In vivo |
|||||
In vivo Formuleringscalculator (Heldere oplossing)
Stap 1: Voer de onderstaande informatie in (Aanbevolen: Een extra dier voor het geval van verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in het gedeelte Oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-mastervloeistof: mg geneesmiddel vooraf opgelost in μL DMSO ( Concentratie mastervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de partij geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toeμL PEG300, mengen en helder maken, voeg vervolgens toeμL Tween 80, mengen en helder maken, voeg vervolgens toe μL ddH2O, mengen en helder maken.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO mastervloeistof, voeg vervolgens toe μL Maïsolie, mengen en helder maken.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysische methoden zoals vortexen, echografie of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Targets/IC50/Ki |
COX2
COX1
|
|---|---|
| In vitro |
Aspirin remt de activering van NF-kappa B, voorkomt zo de afbraak van de NF-kappa B-remmer, I kappa B, en daardoor blijft NF-kappa B in het cytosol. Deze verbinding remt ook NF-kappa B-afhankelijke transcriptie van de Ig kappa-enhancer en de lange terminale repeat (LTR) van het humaan immunodeficiëntievirus (HIV) in getransfecteerde T-cellen. Het en salicylaat worden gedeeltelijk gemedieerd door hun specifieke remming van IKK-beta, waardoor activering door NF-kappaB van genen die betrokken zijn bij de pathogenese van de ontstekingsreactie wordt voorkomen. Deze chemische stof is beschermend tegen neurotoxiciteit veroorzaakt door het exciterende aminozuur glutamaat in primaire neuronale culturen en hippocampale plakjes van ratten. Het activeert de transcellulaire biosynthese van een voorheen onbekende klasse eicosanoïden tijdens co-incubaties van menselijke navelstrengveneusendotheelcellen (HUVEC) en neutrofielen [polymorfonucleaire leukocyten (PMN)]. Dit middel roept een unieke klasse eicosanoïden op die worden gevormd door geacetyleerde PGHS-2- en 5-lipoxygenase-interacties. De behandeling remt de fosforylering van IRS-1 bij Ser307, evenals de fosforylering van JNK, c-Jun, en de afbraak van IkappaBalpha in 3T3-L1- en Hep G2-cellen behandeld met tumornecrosefactor (TNF)-alfa. Deze behandeling remt de fosforylering van Akt en het zoogdierdoelwit van rapamycine (maar niet de extracellulair gereguleerde kinase of PKCzeta) als reactie op TNF-alfa. Het herstelt de insuline-geïnduceerde glucoseopname in 3T3-L1-adipocyten die vooraf zijn behandeld met TNF-alfa.
|
Referenties |
|
Toepassingen
| Methoden | Biomarkers | Afbeeldingen | PMID |
|---|---|---|---|
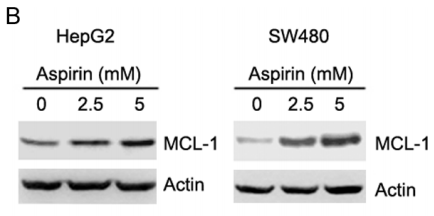
| Western blot | MCL-1 p-AKT / AKT / p-ERK / ERK p-AMPKα / AMPKα / p-ACC / ACC SHH / SMO / GLI1 / Bcl-2 / Foxm1 |
|
26918349 |
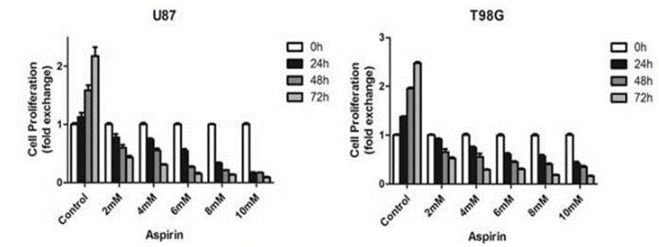
| Growth inhibition assay | Cell proliferation Cell viability |
|
28446712 |
Informatie klinische proef
(gegevens van https://clinicaltrials.gov, bijgewerkt op 2024-05-22)
| NCT-nummer | Rekrutering | Aandoeningen | Sponsor/Medewerkers | Startdatum | Fasen |
|---|---|---|---|---|---|
| NCT05960864 | Not yet recruiting | Ankylosing Spondylitis (AS) / Radiographic Axial SpA (r-axSpA)|Non-radiographic Axial Spondyloarthritis (Nr-axSpA)|Axial Psoriatic Arthritis (axPsA)|Acute Anterior Uveitis (AAU)|Crohn Disease (CD)|Ulcerative Colitis (UC)|Reactive Arthritis (ReA) |
Southwest Hospital China |
September 2024 | -- |
| NCT05868525 | Recruiting | Blunt Cerebrovascular Injury |
Loma Linda University |
July 2024 | Phase 4 |
| NCT06197009 | Not yet recruiting | Axial Spondyloarthritis |
Sinocelltech Ltd. |
July 1 2024 | Phase 2 |
| NCT06378398 | Not yet recruiting | Colorectal Neoplasia |
University of Michigan Rogel Cancer Center|National Cancer Institute (NCI) |
May 2024 | Early Phase 1 |
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, kunt u een bericht achterlaten.
